Abstract
Background:
Black patients are underrepresented in multiple myeloma (MM) clinical trials. Despite the promise of Real-World Data (RWD), little research exists on RWD's usage to address this health disparity. In collaboration with a large pharmaceutical partner, we used RWD from commercial datasets (ConcertAI's Electronic Medical Record and claims datasets) aimed at identifying sites with a large Black patient population. We recommended including these sites in a recent clinical trial of Chimeric Antigen Receptor T cell (CAR-T) therapy for MM patients.
Methods:
We used the following criteria to identify promising sites: (1) high Black patient density, (2) access to a CAR-T accredited parent organization within 100 miles, (3) a hematologist/oncologist who treats MM patients, and (4) a history of treating MM patients with a Proteasome Inhibitor (PI) and Lenalidomide (Len) in the first line of therapy. For (1), sites were ranked using the lower 95% confidence interval for the percent of Black MM patients at the site. For (4), only sites with at least five MM patients who received PI and Len were included.
Our data sources were: ConcertAI's Electronic Medical Record (EMR) and claims datasets to link each patient to a site, and Google maps API to identify the CAR-T center nearest each oncology site. The patients in our data sets were not identifiable, and our research was conducted in compliance with the Health Insurance Portability and Accountability Act.
After having identified and filtered promising sites, we curated individual candidates in the order of Black patient density. The purpose of curation was to validate a final list of sites.
Results:
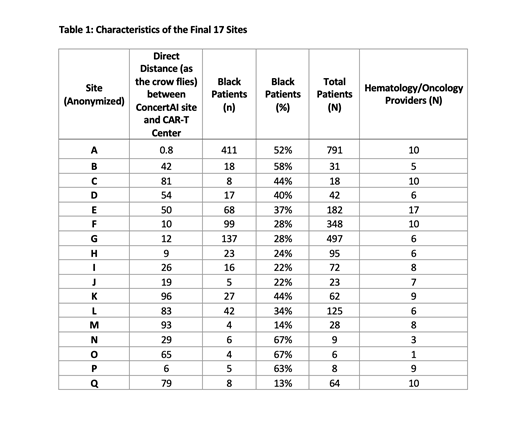
We identified 17 promising clinical trial sites affiliated with 16 healthcare systems in the mid-west, mid-Atlantic, southeastern, and southwestern regions of the United States (table 1). Our RWD captured an average of 141 MM patients (range: 6-791) who were treated at the 17 sites from 2015-2020. Thirty-nine percent of the patients were Black (range: 13-67%). This percentage was three times the recruitment rate of black patients in MM trials in the US (13%). On average, the sites were 44 miles driving distance (range: 0.8-96 miles) from the closest CAR-T center, had eight hematology/medical oncology specialists on staff (range: 1-17), and had previous interventional trial experience (13 sites had experience with MM trials). All of the identified sites were community-based sites, and none of the sites were previously identified by our pharmaceutical partner.
Conclusions:
We demonstrated that RWD can be leveraged to identify clinical trial sites with a high potential for Black patient recruitment, thereby addressing a known health disparity problem within multiple myeloma (MM) clinical trials.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal